The Shortest Timeline to a COVID Vaccine
The response of the scientific community to the COVID-19 pandemic has been remarkable. The NY Times Corona Vaccine Tracker reports 36 vaccines in human studies as of September 1, 2020. To have this many shots-on-goal in less than 9 months is truly amazing and should be applauded.
However, there is a lot of misinformation and hype being spread about when such a vaccine might be available. This information could mislead the public and could even jeopardize our ability to fight the novel coronavirus. The purpose of this article is to lay out some facts and practical considerations for the timing of the Phase 3 clinical trials currently underway.
Everyone wants to develop a vaccine as soon as possible
There are four vaccines that are leading the pack and in Phase 3 clinical studies. The companies sponsoring these studies are Oxford/AstraZeneca, Sinovac, Moderna, Pfizer/BioNTech.
Watch: How researchers will study Covid-19 vaccines (from STAT+)
The clinical study designs for all four vaccines are roughly similar.
The clinical studies are blinded, which means nobody knows which participants receive the experimental vaccine and which receive a placebo. Not the participant, not the investigator who gives the injections, not the healthcare professionals who see these participants. The vials are coded, and the code remains sealed until the data are analyzed.
The first thing we need to do is describe the timeline for a single individual participant in any of the studies. All four vaccine trials follow generally the same pattern.
First Dose, Second Dose, Observe.
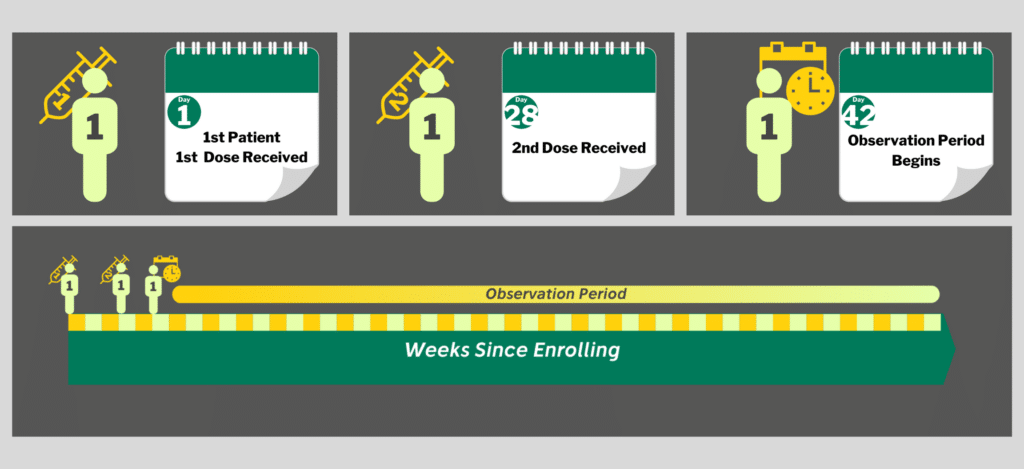
It then becomes a waiting game.
Once a participant has received both doses, they are observed for 1-2 years. If they develop COVID during that time, they are counted as an infection. The numbers of infections are tallied, the rate of infection calculated, and the results are compared between groups at the end of the study.
Dr. Stephen Hahn, Commissioner of the FDA has stated that the FDA will be able to grant Emergency Use Authorization before the end of the Phase 3 Trials. That is absolutely true but the ends of the Phase 3 Trials are in 2022.
The real question is,
“When is the earliest that an Emergency Use Authorization could legitimately be announced?”
To potentially get an answer sooner, the researchers have included interim analyses to take early peeks at the data. The early peeks are only allowed when a predetermined trigger is reached, either a specific number of cases or preset times. When that number is reached, an independent Data Monitoring Committee unseals the code and makes a comparison between groups. The Data Monitoring Committee does not release the data, but they may share conclusions and insights with the study sponsors.
So, let’s make this real
The protocols specify that it will be 1-2 weeks after the first participant receives their second dose before any data can even be collected. This is because it takes the immune system about 2 weeks to build up antibodies. Infections that occur in those first two weeks probably were already started before the vaccine was delivered. The dates of the first participants’ second doses in these Phase 3 clinical studies are listed below[i] [ii]
| PROGRAM | SECOND DOSE |
|---|---|
| Oxford/AstraZeneca | June 28 |
| Sinovac | Aug 5 |
| Pfizer/BioNTech | Aug 17 |
| Moderna | Aug 24 |
Oxford/AstraZeneca may have had a few cases dribbling in but other trials may not have even reached the observation period. Bottom line: There are very little data to even look at yet. Not even for the very first participants.
These studies include as many as 30,000 participants.
Why so many?
There needs to be enough cases to show a meaningful difference between experimental vaccine and placebo. Furthermore, there needs to be enough cases to have confidence in the result. In general terms, hundreds of cases will need to accumulate before an interim analysis (early peek) can be done.

It is hard to tell exactly how many cases are needed. The sponsor companies have not released their detailed plans for the interim analyses. However, we can see some hints based on the data published in ClinicalTrials.gov
- Sinovac is studying 8870 health care workers at a high risk of infection. They state that they will need 150 cases out of this sample for an interim analysis.
- Pfizer/BioNTech is going to look at acute reactions (a safety measure) when 360 people are enrolled. The next number they mention is 6000 participants, again for safety data. Both of these milestones may provide opportunities to look at efficacy.
When will they accumulate enough cases?
No. One. Knows.
However, common sense reveals that this will be months at best. For any given participant, it will take at least 6 weeks after the second dose until they are confirmed as having COVID-19. They need to be exposed, get the infection, develop symptoms, and get tested.
Which brings up another practical matter. It takes time to recruit, enroll, and vaccinate participants. Moderna enrolled 13,000 of the 30,000 they need in about a month. A reasonable, conservative assumption is two months to reach full enrollment. That averages to about 500 enrolled each day.
The US has averaged approximately 41,000 new cases daily for the past week (August 25 – September 1). That translates to 13 new cases per 100,000 population each day. If those rates hold, we would expect approximately 4 new cases per day on average for our group of 30,000… if nobody gets the vaccine. If the vaccine is working as expected, the number of new cases per day will be even less, and the time to reach the predetermined number of cases will be longer.
Given all this practical information, we can make an estimate of the absolute earliest that an interim study result could be available after the second dose. If we add the 8 weeks to recruit the study and the 6 weeks for detecting infection, the total is 14 weeks (about 3½ months.) The projections for the four lead vaccines are listed below.
| PROGRAM | SECOND DOSE | FIRST POSSIBLE INTERIM |
|---|---|---|
| Oxford/AstraZeneca | Jun 28 | Oct 15 |
| Sinovac | Aug 5 | Nov 20 |
| Pfizer/BioNTech | Aug 17 | Dec 1 |
| Moderna | Aug 24 | Dec 8 |
This means that only Oxford/AstraZeneca could have a chance of an interim readout in October. However, AstraZeneca has indicated that they are not even in talks with FDA about Emergency Use Authorization.[iii]
The work is not done when the magic number of cases is reached
The data will need to be reviewed by the Data Monitoring Committee, internal decisions will need to be made, and the application for Emergency Use Authorization will need to be written and submitted to the FDA. This is not a trivial task when you consider the amount of data that needs to be analyzed. If 30,000 are enrolled, 30,000 will need to be submitted, at least for safety.
Once all these data are compiled. The scientists need time to think about what they mean and how to state their risk-benefit argument for authorization. We cannot just assume the best-case scenario. At least two of these experimental vaccines (Moderna, Pfizer/BioNTech) are using groundbreaking novel mRNA technology. The promise of this technology is too important to do a shoddy job assessing the risks and benefits just to meet an externally imposed timeline.
FDA Review Must Not Be Compromised
Finally, the FDA will need to perform a scientific and medical review of the application. This should not be a cursory review. They must digest the data and be certain that the vaccine is safe and effective enough to release under Emergency Use Authorization. They must base their decisions on the facts and data presented.
- As mentioned earlier, some of the vaccine technologies are new. Safety is not guaranteed.
- Our understanding of the novel coronavirus continues to evolve. Efficacy is not guaranteed either.
- Several companies are racing to the goal. But the first may not necessarily be the best.
Lowering the standards too far from the Guidance for COVID Vaccine Approval that FDA published on June 30, 2020[iv] could (and should) undermine the American Public’s trust in the decision.
And that may leave us with an under-vaccinated population.
So, what should you believe?
Dr. Anthony Fauci has consistently said that he is cautiously optimistic that a vaccine will be available by late 2020 or early 2021. Given the facts on the ground, the latter seems much more probable but far from certain.
Disclaimer: All views are the author’s. Projected dates are estimates best on educated and informed estimates. Only publicly available information has been used to develop this assessment. The author was formerly employed at Pfizer and holds options on PFE.
[i] https://clinicaltrials.gov “Actual Start Date” except for Pfizer/BioNTech, taken from NEJM article below.
[ii] https://www.nejm.org/doi/full/10.1056/NEJMp2027405?query=TOC
[iii] https://www.fiercepharma.com/vaccines/astrazeneca-not-involved-covid-19-vaccine-emergency-use-authorization-talks-spokeswoman
[iv] https://www.fda.gov/regulatory-information/search-fda-guidance-documents/development-and-licensure-vaccines-prevent-covid-19
Posted by Kevin Freiert
[social_warfare]